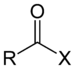
Acid -Base Tritation
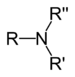
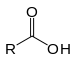
It is the branch of analytical chemistry in which acid react with base or alkali or vice versa.Acid When acid disslove in water it give Hydrogen ion.
HA---------------------H + A
Base When base disslove in water it give Hydroxyl ion.
BOH-------------------------------B + OH
When they react with each other , the reaction occuring is,the neutralization of hydrogen ion with hydroxyl ion to form unionized water.
H + A + B+OH------------------B + A + water
H+OH----------------------------------Water
Indicators
Indicator is a substances with is used for the visual detection and determination oa a specfic consitutient present in any sample.Visual detection is a primarily color , but observations of florescence and turbidity are also used.
Indicator used in all reagents such as Colorimetry Florimetry Turbidimetry.
Acid base indicator Acid base indicator are organic substance in which acid solution have one color while base or alkaline solution gives different colors.